The fog of pseudoscience surrounding the microbiome has finally been lifted and approval for the first live bacterial therapeutic is now on the horizon. Dr Michelle Tempest and Isabel Brooks at Candesic look at how bacteria are set to become the next big break-through in the life sciences
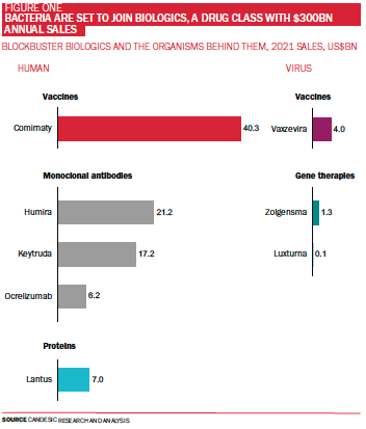
Mother nature has inspired what is arguably pharma’s most exciting market: biologics ‒ therapeutics derived from living organisms. Biologics are valued globally at over £200bn and sales have grown by over 75% in the last decade (see Figure One). While human biology has provided inspiration for an overwhelming majority of blockbuster biologics to date, viruses have formed the backbone of key drugs. Viruses can take credit for gene therapies in retinal and spinal muscular atrophy, blood disorder beta thalassemia, as well as a cancer-killing melanoma therapy, and vaccines against infectious diseases including Covid-19.
Bacteria, another class of microbe, haven’t yet provided us with any approved treatments to date, but the tide is finally set to turn.
What is the microbiome and why is it important?
Although we don’t currently have any bacteria-based treatments, that is not to say these organisms don’t already play a key role in human health ‒ 90% of cells on and inside the human body are in fact bacterial. These vast numbers of microbes assemble into a community, known as the microbiome, on every surface of the body with key implications for health and disease.
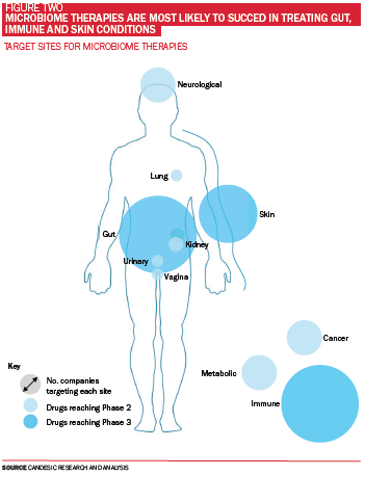
To date, much microbiome science has been centred around the gut. The average adult gut has a surface area of 32 meters squared which enables trillions of bacteria to colonise and interact with human cells in ways that shape our immune and nervous systems. Conditions from allergy and inflammation, to depression via the ‘gut-brain axis’ and even ageing are now thought to be influenced by our own body’s bacteria. Nevertheless, our understanding of the role of bacteria on other sites – from skin on the outside to respiratory, urinary and reproductive tracts on the inside – is also accelerating (see Figure Two).
An emerging field
Scientists have only just started to understand the enormity of this sector. This year marks the tenth anniversary of the Human Microbiome Project (HMP), a gene sequencing effort which depicted, for the first time, the diverse bacterial community living in the human gut. Before gene sequencing, studying bacteria meant growing them in Petri dishes ‒ an unsuitable approach given that most gut bacteria die upon exposure to oxygen. Thanks to HMP and the cost of sequencing dropping, researchers have successfully characterised diverse microbiomes.
Real help or hype?
Some commercial players in the food and wellness industry were quick to hype their products as ‘gut-friendly’ ahead of any academic rigour or evidence base. Yoghurts, other fermented food and probiotic capsules tried to boost sales by jumping on exaggerated, unproven claims about weight loss and boosting the immune system. Currently, no probiotics have been clinically approved to treat or prevent disease. Even the notion of taking probiotics after a course of antibiotics ‒ the logic being that antibiotics wipe out the gut microbiome, so probiotics help re-establish it – is now being reconsidered.
Thankfully, the microbiome’s credibility as a therapeutic tool is set to recover with the first live biotherapeutic product (LBP) poised to receive approval by the US Food and Drug Administration (FDA). In June 2022, Seres Therapeutics announced positive results of its final trial stage for microbiome-based SER-109 to treat Clostridium Difficile (C.Diff), a life-threatening condition associated with diarrhoea, fever, nausea and abdominal pain. Finally, a rigorous clinical evidence base distinguishes credible microbiome-based therapeutics from the pseudoscience.
Working beyond the gut
Bacteria are also increasingly understood to have wider-reaching effects. Around 15‒30%of active biological molecules (metabolites) found circulating in the blood come from bacteria, which is strong evidence to suggest they have systemic impacts on health.
Arguably the most promising and wide-reaching effects of LBPs are emerging in oncology. The success of a transformational immunotherapy – checkpoint inhibitors – is now strongly evidenced to be controlled by gut bacteria. The two best-in class checkpoint inhibitors, Keytruda (Merck) and Opdivo (Bristol-Meyers Squibb) are predicted to become the two top-selling pharmaceutical products by 2026 – despite only 10‒45% of patients responding to therapy. Turning non-responders to responders is a top priority, and the microbiome has emerged as a key tool to do so. Earlier this year, Microbiotica’s MB097, a multi-strain drug consisting of nine species shared across therapy respondents, was effective in pre-clinical trials.
Systemic conditions related to the immune system are also likely to benefit from LBPs. French biotech MaaT Pharma has made strong progress using the microbiome to fight graft-vs-host disease (GvHD), a severe complication affecting up to 50% of stem cell transplant recipients in which transplanted immune cells attack the transplant recipient.
Currently in Phase 3 trials, multi-strain MaaT013 aims to regulate the attacking donor-derived cells by replicating the donor’s microbiome in the transplant recipient.
Single bacteria strains for skin and female health
Outside the gut, microbiomes are less diverse and single strains of bacteria have fared better in trials. Lower diversity among species may mean less variation between individuals, creating a higher chance that a given strain will have a uniform effect across individuals. Boston-based biotech AOBiome is developing a single-strain topical skin therapeutic for atopic dermatitis. Rather than looking at patterns in the human biome, AOBiome took an alternative approach and looked to one of the richest microbiomes on earth – the soil. AOBiome’s LPB is a single strain extracted from soil and strongly evidenced to reduce inflammation. It is soon to enter Phase 3 trials for the treatment of eczema and prurius (itch).
There has also been a steep learning curve regarding the importance of vaginal microbiome. The current industry focus is bacterial vaginosis (BV), a common imbalance of the vaginal microbiome which, if untreated, can cause infertility. LUCA Biologics, founded by a leading researcher in the vaginal microbiome field, is targeting BV as well as urinary tract infections and pre-term birth, with its first therapeutic candidate set to enter clinical trials later this year.
FemTech is also targeting consumer-centric technologies to measure vaginal microbiome. One example is UK-based start-up, JunoBio, which offers home testing for the vaginal microbiome, backed by large company Illumina. Another start-up, Daye, is developing a screen for vaginal biome imbalances. To date, the tests are purely a commercial offer, but both are working on a regulated version to inform clinical treatment decisions.
‘Disruptions to the vaginal microbiome have been associated with a number of adverse gynaecological health outcomes, including an increased risk of contracting sexually transmitted infections and of experiencing a miscarriage,’ said Valentina Milanova, Daye founder and CPO. ‘Daye’s tampon-based vaginal microbiome screen is set to be released this September. Customers simply return their tampon back to one of our partner laboratories for a full vaginal microbiome screening, without the need to attend a specialist appointment. Our aim is to make educational, digestible, and actionable insights into gynaecological health readily available for all.’
Platforms for big data analysis
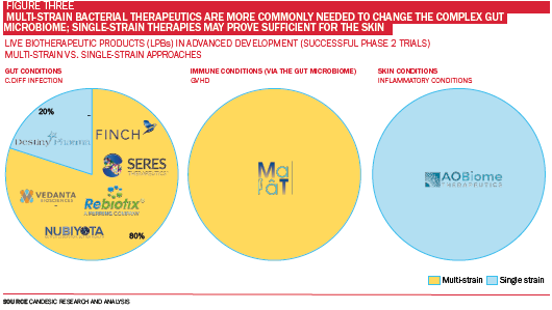
It perhaps comes as no surprise that Artificial Intelligence (AI) is helping accelerate the pace of discovery by allowing scientists to identify cocktails of bacteria with therapeutic potential. Candesic research suggests that 80% of LBPs in Phase 3 clinical trials for gastrointestinal conditions are ‘multi-strain’ drugs, meaning more than one type of bacteria is administered (see Figure Three). The leading candidates for C. diff infection developed by Seres Therapeutics, Finch Therapeutics, Rebiotix & Vedanta Biosciences have all used multi-strain approaches.
‘The microbiome is a complex and personalised community. Which species can survive and thrive relies on many factors and can vary across individuals,’ said Dr Sioned Jones, COO at Booby Biome, a start-up researching how bacteria found in breast milk contribute to infant gut health. ‘With this in mind, single-strain products may not translate well from person-to-person, whereas multi-strain approaches provide a higher likelihood of survival and colonisation.’
The key to developing multi-strain drugs lies in leveraging insights from big data and AI. In human studies, where each person possesses thousands of varying bacterial strains in their gut, the challenge is to identify the combination of bacterial strains that consistently correlates with better clinical outcomes. For companies developing drugs to modulate the gut microbiome, proprietary data and processing platforms will largely determine success.
Companies offering such information and number crunching ‘platform technologies’ have taken over two thirds of global biotech funding between 2019 and 2021, offering to deliver return on investment with an increased chance of launching several successful therapies from the same platform.
To tackle the immense quantity of data, the team at BioCorteX leverages approaches typically used in aerospace and engine design. ‘The microbiome represents one of the most complex mathematical problems in health requiring entirely new approaches to deliver effective therapeutics and real value,’ said its CEO Nik Sharma.
Engineered bacteria – the future?
Engineered bacteria are controversial. However, consistent clinical trial activity is testament to the interest in these therapies. In the wider biologics field, engineering has been the key to enhancing what mother nature has to offer. From monoclonal antibodies to gene therapies, our key biologics are the result of powerful editing to target disease in a more powerful, specific manner. To date, the overwhelming majority of microbiome therapies under development use ‘naturally-occurring’ strains, due to the difficulty of safely engineering bacteria.
Regulating bacteria can be challenging, as it’s difficult to control how they spread their DNA.
Microbes constantly share DNA with one another. Engineering bacteria by adding ‘new’ DNA will inevitably cause the ‘new’ DNA to be used by other bacteria. With the gut microbiome playing host to thousands of species, it’s hard to predict how each one of these species will use this ‘new’ DNA. And for this reason, it is challenging to confirm engineered bacteria are safe.
Companies are nevertheless striving to bring engineered bacteria to market. Under strict regulation, engineered bacteria are permitted for use in clinical trials so long as they demonstrate safety modifications. Actym Therapeutics is developing a first-in-class immunotherapy in the form of an attenuated strain of Salmonella, engineered to release molecules which activate the immune system to fight cancer. Novome Biotechnologies is a few steps ahead, planning Phase 2 trials for a proprietary strain engineered to degrade oxalate, a normal dietary molecule which can lead to end-stage renal failure in patients with enteric hypoxaluria, a frequent complication of gastric bypass.
Conclusion: the promise of bacteria
It’s clear that microbiome-based therapeutics have a great deal of promise. Multi-strain approaches will continue to provide the most exciting breakthroughs in the gut, with single-strain microbiome treatments showing promise for the skin and vagina. Overall, evidence is mounting fast for the newest class of biologics to help solve some of human health’s biggest challenges. Candesic sees exciting opportunities for investors as the world races towards understanding the promise of bacteria living within all of us.







 ©2024 All rights reserved LaingBuisson
©2024 All rights reserved LaingBuisson